Bisphenol A - Overview
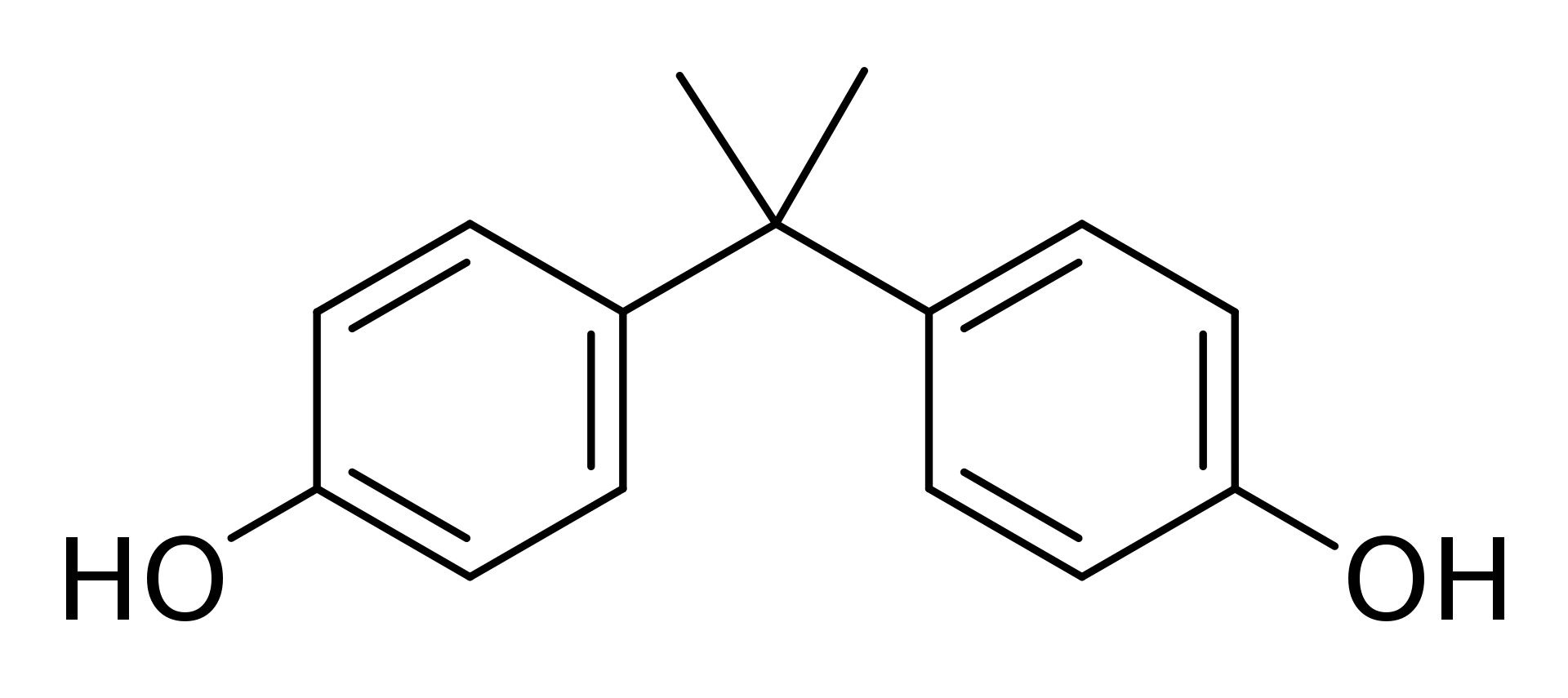
Bisphenol A is a chemical compound containing two phenol functional groups. The chemical formula of Bisphenol A is C15H16O2. It belongs to the phenol class of aromatic organic compounds. It is prepared by the reaction of two equivalents of phenol with one equivalent of acetone. Bisphenol A was first synthesized by A.P. Dianin in 1891. Bisphenol A was investigated in the 1930’s during the search for synthetic estrogens. At that time, another synthetic compound, diethylstilbestrol, was determined to be more powerful than estrogen itself, so bisphenol A was not used as a synthetic estrogen. It’s current uses are as a primary monomer in polycarbonate plastic and epoxy resins.
Manufacturing Process
This compound is synthesized by the condensation of acetone with phenol. The reaction is catalyzed by a strong acid, such as hydrochloric acid (HCl) or a sulfonated polystyrene resin. Industrially, a large excess of phenol is used to ensure full condensation; the product mixture of the cumene process (acetone and phenol) may also be used as starting material. A large number of ketones undergo analogous condensation reactions. Commercial production of BPA requires distillation – either extraction of BPA from many resinous byproducts under high vacuum or solvent-based extraction using additional phenol followed by distillation.
The Uses of Bisphenol A
Polycarbonate Plastics
Bisphenol A (BPA) is a chemical building block that is used primarily to make polycarbonate plastics. Polycarbonate plastic is a lightweight, high-performance plastic that possesses a unique balance of toughness, optical clarity, high heat resistance, and excellent electrical resistance. Because of these attributes, polycarbonate is used in a wide variety of common products including digital media (e.g., CD’s, DVD’s), electrical and electronic equipment, automobiles, sports safety equipment, reusable food and drink containers, and many other products.
Epoxy Resins
PA is also used in the production of epoxy resins. Epoxy resins have many uses including engineering applications such as electrical laminates for printed circuit boards, composites, paints and adhesives, as well as in a variety of protective coatings. Cured epoxy resins are inert materials used as protective liners in metal cans to maintain the quality of canned foods and beverages, and have achieved wide acceptance for use as protective coatings because of their exceptional combination of toughness, adhesion, formability, and chemical resistance.
Pharmaceutical Industry
Polycarbonate plastic is used to make critical components of many medical devices and their housings. Its optical clarity allows direct observation of blood or other fluids to monitor proper flow. Health care providers depend on medical devices and equipment made with BPA for a transparent view within the human body so they can check for the presence of air bubbles or other obstructions during medical procedures.
Construction Industry
BPA is regularly used to strengthen products for human health and safety. Products like bike helmets, police shields, reading glasses and bullet-proof glass are all shatter resistant because of BPA.
